Fact Sheet
COVID-19Health in School
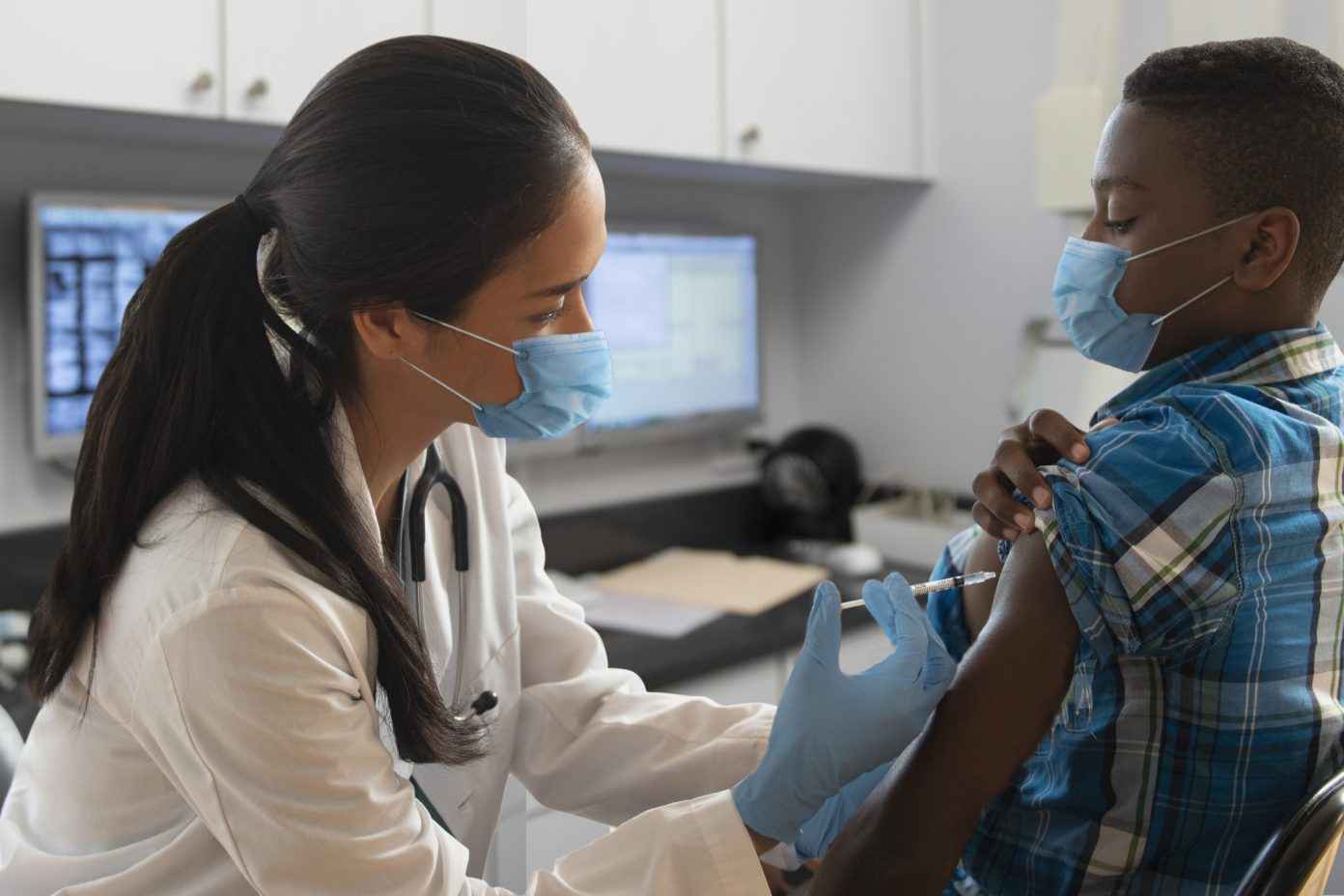
COVID-19 Vaccination: State Minor Consent Requirements
June 15, 2021
Overview
Pfizer-BioNTech’s COVID-19 vaccine has been authorized for emergency use in minors between the ages of 16 and 18, and more recently, for minors between ages 12 and 15. As a result, minors across the country have been getting vaccinated, and accompanying questions have arisen regarding minor consent. This fact sheet provides a review of select state requirements, and additional information as available from certain state or local health departments.